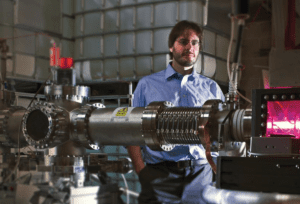
Greg Piefer, SHINE Medical Technologies
NorthStar Medical Radioisotopes, one of two Rock County companies developing radioactive isotopes for medical imaging and therapies, said the Food and Drug Administration has approved its new method of making a key isotope that can increase production fourfold.
The other company, SHINE Medical Technologies, said it broke ground last month on a 54,000-square-foot facility that will house its corporate headquarters and production of therapeutics.
The companies are developing facilities to make molybdenum-99, or Mo-99. The isotope decays into technetium-99m, which is used to detect cancer, heart disease and other conditions in about 80 percent of nuclear diagnostic imaging procedures, or about 40,000 procedures in the U.S. every day.
The companies are vying for a resurgent domestic production market of the time-sensitive isotopes after a severe shortage a decade ago brought to light the vulnerability of relying on a few aging nuclear reactors outside of the country.
Federal legislation in 2013 made money available to encourage American companies to get into the business.



